Design Controls Process Design Controls For The Medical Device Industry The Ultimate Guide To Design Controls For Medical Device Companies Iso 13485 Design Control Gooluda Read Design Controls Risk Management Process Validation For Medical Bill Fry Design Build Process And Automation Solutions TM Process Design Controls are practices required by the FDA to facilitate a structured design and development process for medical devices There are multiple checkpoints in place to ensure the device is safe and effective when brought to market If you are a medical device manufacturer compliance with Design Controls is required
Design controls are a set of quality practices and procedures that are incorporated into the product design and development process to ensure that a device is appropriate for its intended use 1 Design Controls not only help achieve regulatory compliance they help develop better products Many companies think that design controls are a burden to development organizations imposed by the FDA and it s the price to pay for playing in the medical device field
Bill Fry Design Build Process And Automation Solutions TM Process Design Controls Definition Arena Lack Of Systemic Evaluation And Financial Resources Www Design Controls For Medical Devices Depicted As Waterfall Design 5 Errors To Avoid When Implementing Iso 13485 Flowchart Management System SMMMedyam How Design Controls Apply To The 510 k Process All Roads Lead To AWS 4 Security By 2B Adrian LeGaspi Design Build Process And Automation Solutions TM
Design Controls Process
 Design Controls Process
Design Controls Process
http://www.mdapprovals.com/traceabilityChart.jpg
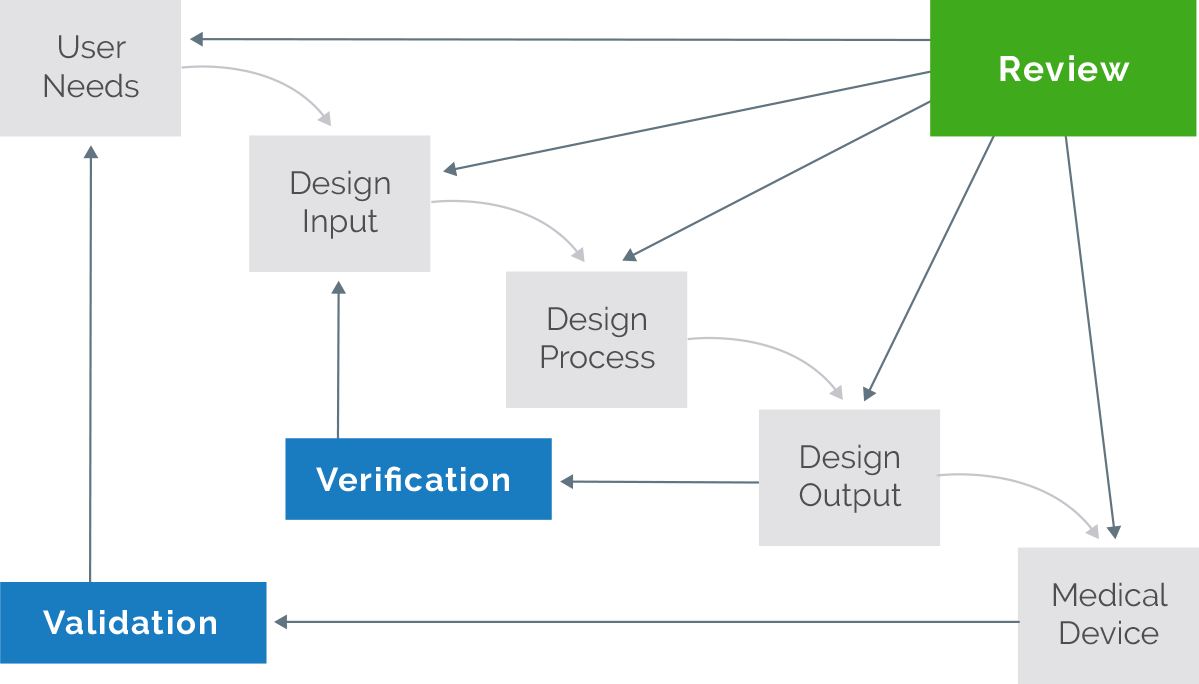
Design control starts with the development and approval of design inputs and includes designing a device and the associated manufacturing processes Design control focuses on all aspects of designing a product and the implementation of the design Some of the tools and techniques that can be used are described in the guidance
Pre-crafted templates offer a time-saving option for developing a diverse variety of documents and files. These pre-designed formats and layouts can be used for various personal and professional projects, consisting of resumes, invitations, flyers, newsletters, reports, discussions, and more, improving the material development process.
Design Controls Process
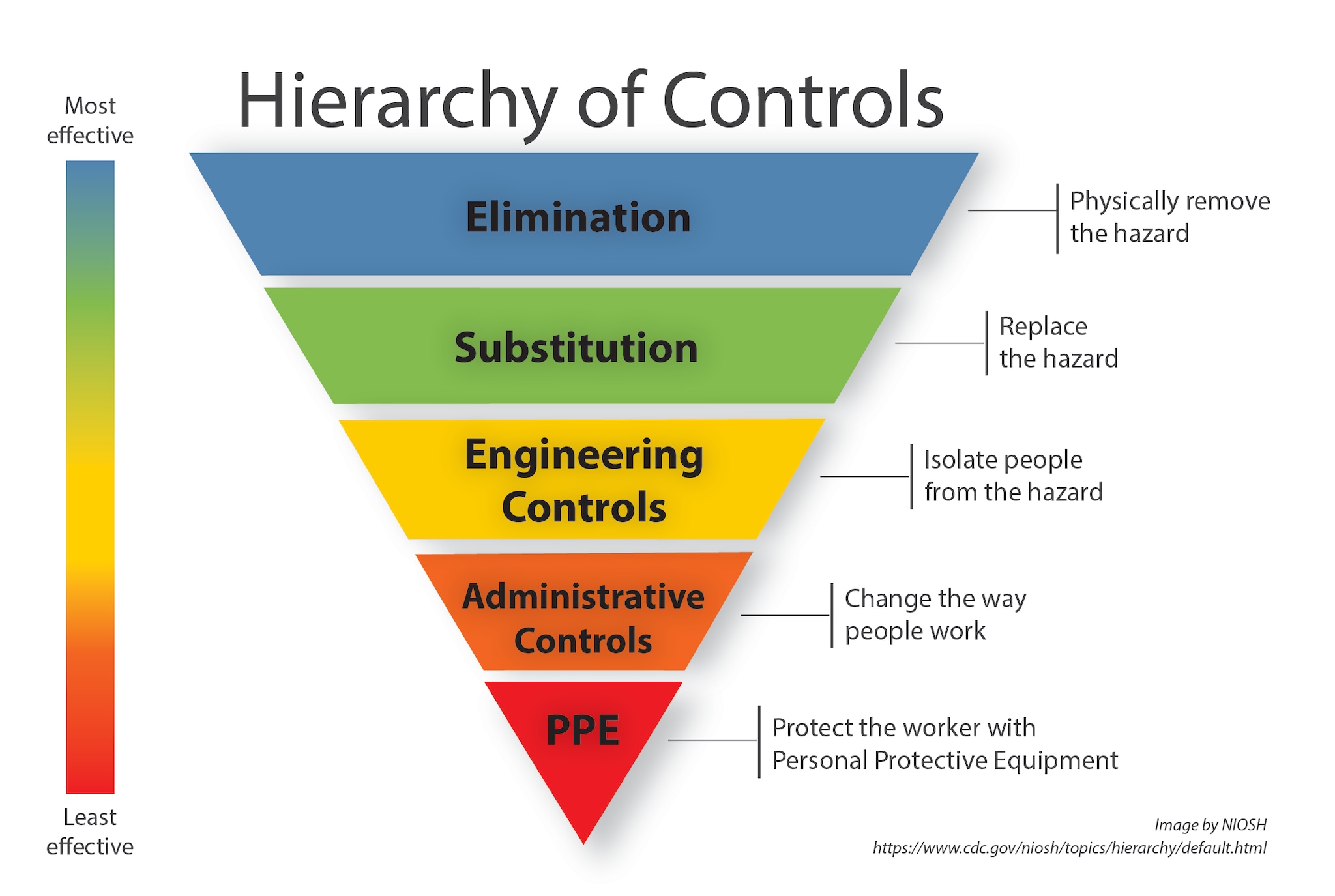
Hierarchy Of Controls Eustaquio Diniz
Design Controls Definition Arena
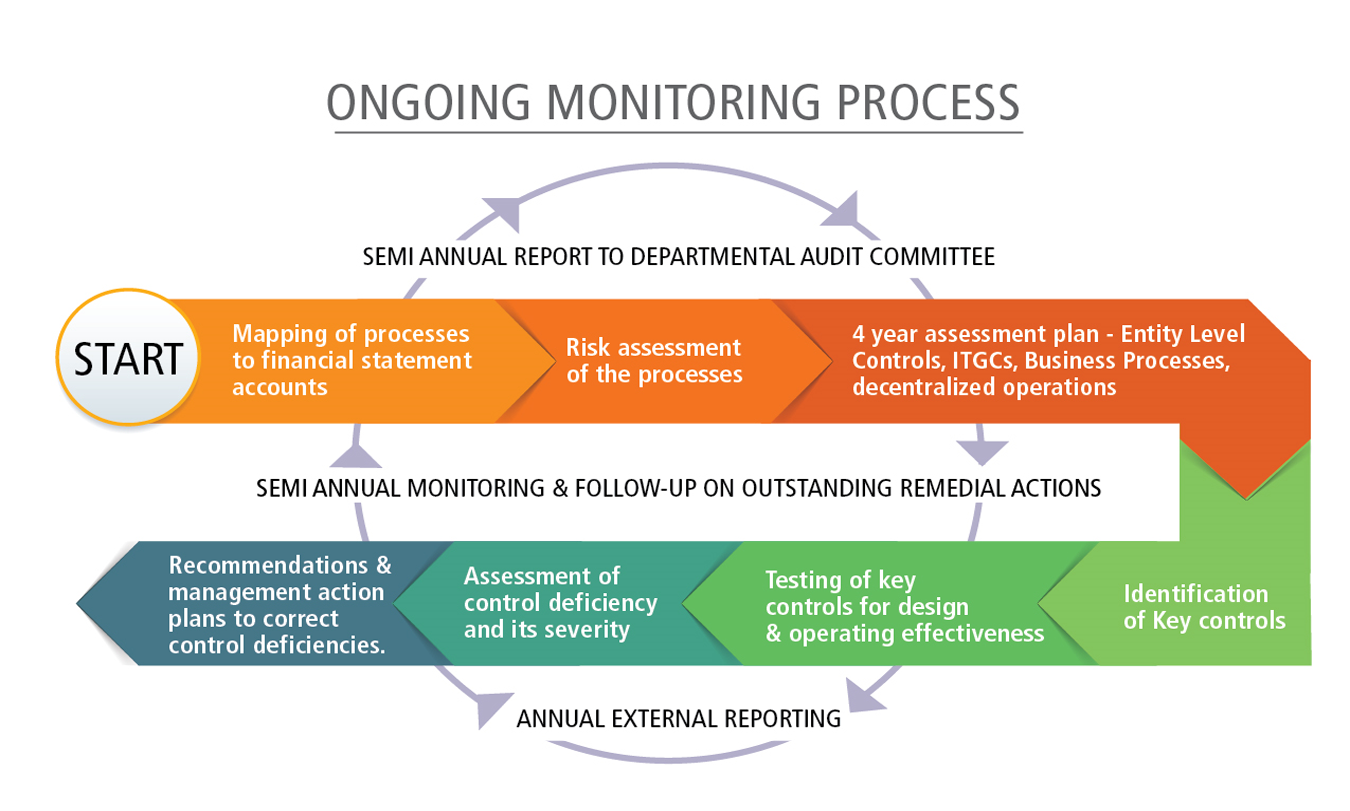
Lack Of Systemic Evaluation And Financial Resources Www

Design Controls For Medical Devices Depicted As Waterfall Design
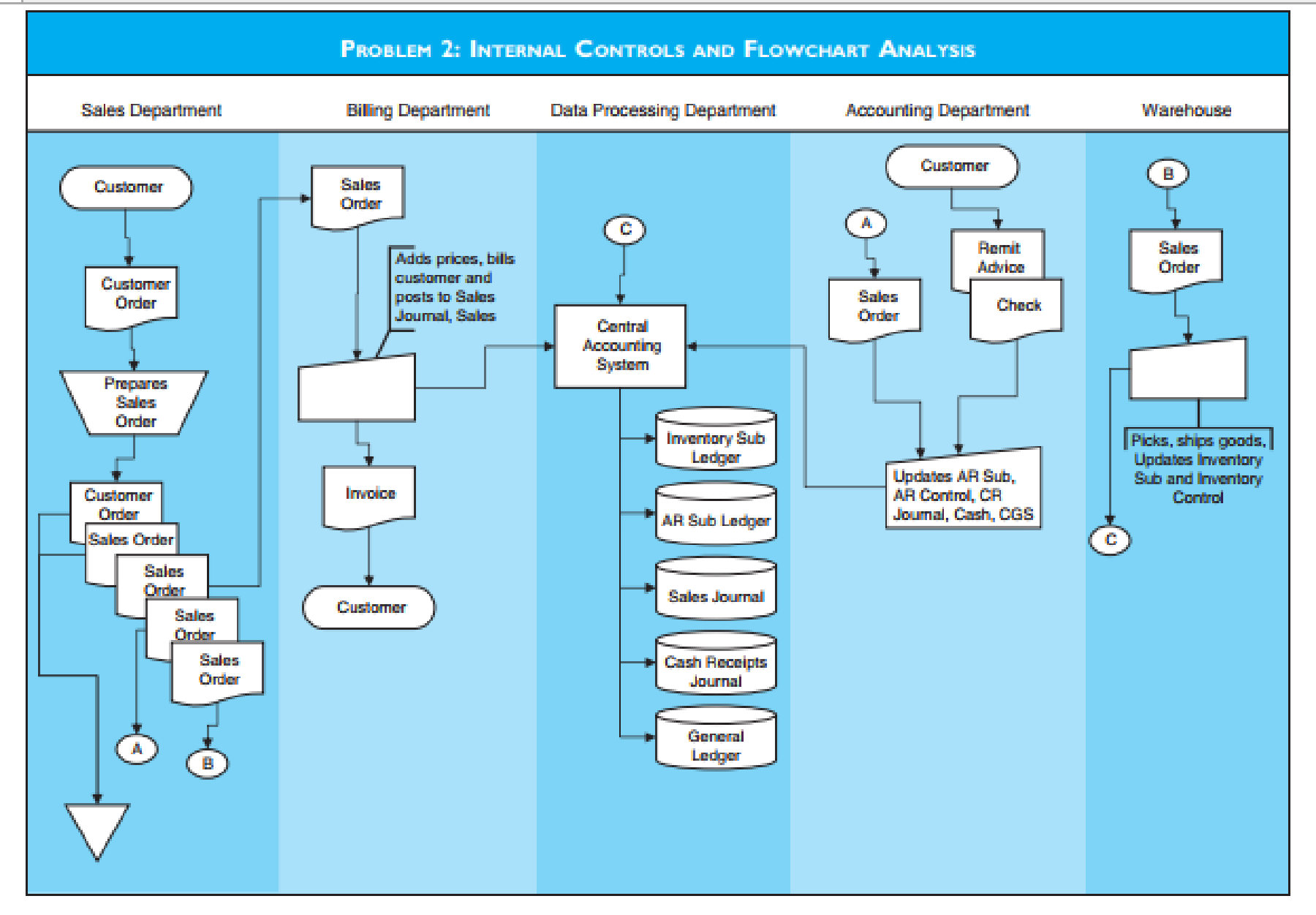
Flowchart Management System SMMMedyam

How Design Controls Apply To The 510 k Process

The objective of Design Controls in this context is to require that manufacturers follow a methodologically sound process to develop a medical device with the intent of improving the probability that the device will reach an acceptable level of efficacy and safety Design input Examples of design input 1 References and external links

1 Select a single design project Note If the project selected involves a device that contains software consider reviewing the software s validation while proceeding through the assessment of

Design Controls Digest 5 Phases of Design Controls You Need to Know February 11 2021 MedTech Compliance Regulatory MedTech Guidelines Standards So you have an idea for a medical device and you obviously want to take it to the market so that it can have a positive impact on tons of people s lives and maybe make you a little money as well

Control the design process to assure that the device meets User needs Intended uses Specified requirements Can improve and prevent future issues Design Controls Why 44 of voluntary

Design Controls which are mandated by the FDA represent a formalized approach to the development of Class II and Class III medical devices This process includes many layers of required documentation that show the FDA exactly how you have provided for the safety and efficacy of your new device
The first step of implementing design controls is to create and design controls procedure You will also need at least two of the following additional quality system procedures Risk Management Procedure SYS 010 Software Development and Validation SYS 044 Usability Procedure SYS 048 Cybersecurity Work Instruction WI 007 3 Design Controls What are they A set framework of quality practices and procedures incorporated into the design and development process Control the design process Premarket and
An introduction DESIGN PLANNING Resources timelines and scope what are you developing and how THE EXTENT OF DESIGN AND DEVELOPMENT PLANNING SHOULD REFLECT COMPANY SIZE AND COMPLEXITY AND ANY OUTSOURCING Refining is OK especially for new portfolio products Identify key milestones and dates only Detail should be dependent on risk